INTRODUCTION
Matter- The term matter refer to anything that occupies space and has mass. All matter is made up of substance called element which have specific chemical and physical properties and cannot be broken down into other substance through ordinary chemical reaction.
Molecule- A group of atom bonded together, representing to the smallest fundamental units of chemical compound that can take part in chemical reaction.They are made of two or more atom join together can be the same or different element.
Atom- Atom is the smallest particle of element which take part in chemical reaction. Atom are the basic unit of matter and they define structure of element. Atomic particlei.Protonii.Neutroniii.Electron Atom consists of electrons surrounding a neutron that contains proton and neutron. Neutron are electrically charge ,proton are positively charge and electrons are negatively charge.
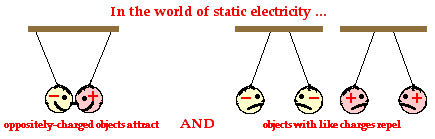
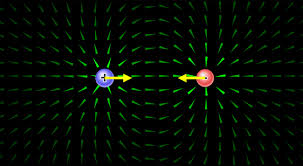
Electric charge- Electric charge is the physical property of matter that causes it to experience a force when placed in an electromagnetic field. There are two types of electric charge: positive and negative. Like charges repel each other and unlike charges attract each other.
A person who walk across a carpet and then touches a metal doorknob might receive a small shock. A basic property of matter, electric charge is a quantity of electricity that is related to the balance of electron and protons in an object. The electric charge is measured(SI unit) using coulomb is exactly 1.602176634×10−19 C. 1 coulomb is the quantity of charge transferred in one second.
Given by: Q = I.t